Marangoni Bursting
In this blog post, I will introduce a visually stunning effect called Marangoni bursting, explain the science behind it, and show how you can recreate this effect easily and cheaply.
A review of surface tension
Surface tension is created by the cohesive forces of neighboring fluid particles at an interface and has a tendency to minimize surface area. A classical example of surface tension gradients is the meniscus formed at the boundary of a test tube and water. A concave meniscus is formed since the adhesive forces between the glass and water molecules at the boundary are greater than the cohesive forces between neighboring water molecules away from the boundary. Conversely, mercury in a test tube forms a convex meniscus as the cohesive forces between the liquid are stronger than the adhesive forces at the boundary of the test tube.
Introduction to Marangoni flows
Marangoni flow is a fluidic effect driven by the gradient of surface tension between two fluids. As from above, surface tension describes the intensity at which one type of liquid pulls on its neighboring molecules. Hence if there is an interface of two liquids with different surface tensions, one liquid will pull more strongly on the other liquid. This imbalance of forces leads to Marangoni flow.
To demonstrate this visually, fill a shallow dish with water and grind pepper flakes on the surface. Using a toothpick deposit a small drop of dish soap in the center. The pepper flakes are instantly pulled outwards! This can be explained through a difference in surface tensions: water has a higher surface tension than dish soap. As such the water molecules exert a great force on their neighbors (in this case the pepper flakes). Before the dish soap is added the system is in static equilibrium: the water evenly pulls on each pepper flake in all directions. After adding dish soap there is an imbalance of forces (weaker forces pulling to the center and stronger forces pulling outwards), this imbalance drives the pepper flakes radially outwards. This is known as a Marangoni flow.
Similar results can be achieved by filling a dish with milk and several drops of food coloring then depositing a small drop of dish soap. The physics describing this system is the same as the water and pepper flakes.
Prior to dish soap being added the pepper flakes are held in static equilibrium. After the soap is added (as in the picture) Marangoni flows push the pepper outward.
A review of vapour pressure
(we're almost at the good stuff!)
Marangoni flows are fascinating in their own right, but before getting to the unique case of Marangoni bursting I must first review vapour pressure. Vapour pressure is the pressure a gaseous vapour exerts on its liquid counterpart when the system is in thermodynamic equilibrium. If two liquids (say, water and isopropyl alcohol) are left open at standard temperature and pressure, their difference in vapour pressure can explain the difference in rates of evaporation.
Isopropyl alcohol (IPA) has a much higher vapour pressure than water. Since IPA requires a higher pressure for an equilibrium between liquid and gas volumes to be maintained the liquid IPA will evaporate at a faster rate than water. A further discussion can be had, however, only the fundamentals of vapour pressure are required to explain the difference in evaporation rates in the context of this experiment.
Marangoni Bursting
Fill a flat, shallow dish with cooking oil to a height of about 1cm. Separately mix water, isopropyl alcohol, and food colouring, while I encourage independent experimentation a good starting point is a ratio of 3ml to 1.5ml to 2 drops of the three liquids, respectively. Use a syringe to deposit a small amount of this mixture on the bed of oil. The effect will begin instantly, sit back and enjoy the mesmerizing show.
Tips:
Isopropyl alcohol evaporates rapidly, thus if you wait between mixing and depositing the mixture on the oil, the concentration of alcohol will be different from when it was initially created the mixture.
If you are doing repeated trials, I found that to save oil you can briefly lay a paper towel on the surface to absorb the water and ethanol droplets while keeping most of the oil in the bottom of the dish for the next trial. While I didn’t test it, this could also be a unique way of making tie-dye shirts.
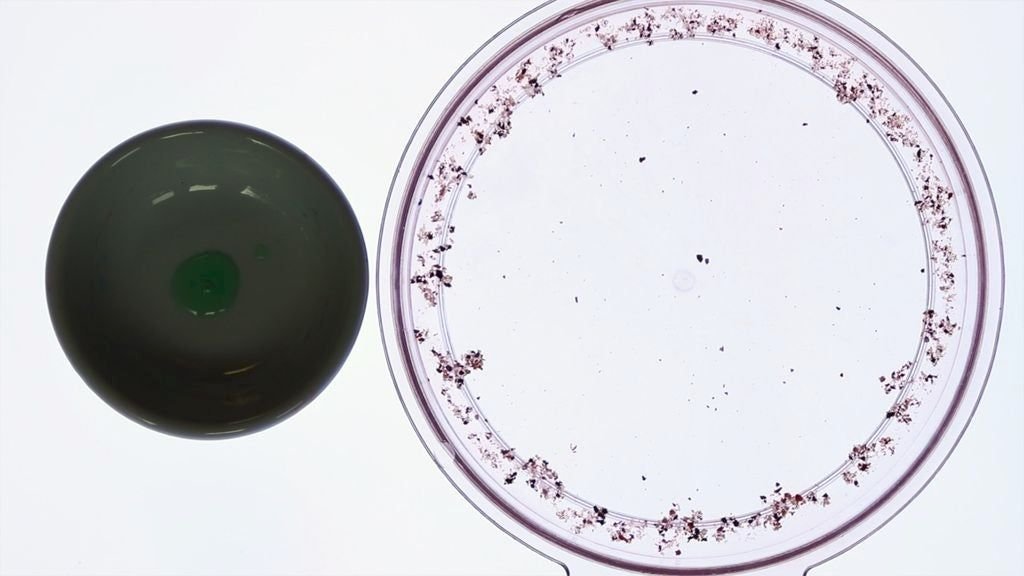
Marangoni bursting
An explanation of Marangoni bursting
Isopropyl alcohol has a lower surface tension than water and higher vapour pressure, meaning it evaporates much faster. Thus, at the edge of the droplet the alcohol evaporates quickly resulting in a lower alcohol concentration at the edge relative to the center. This lower concentration of alcohol means that the surface tension at the edge is stronger and so the interior mixture is pulled outwards via Marangoni flows. This cycle repeats with more and more of the mixture collecting at the outer rim.
As liquid collects at the outer rim, surface perturbations create instabilities and long fingers begin to protrude radially outwards. These fingers break into tiny droplets that shoot radially outwards. Together, the differences in surface tension and vapour pressure result in the stunning creation of hundreds of smaller droplets.
Going Further
I encourage you to consider how the principles learned above can be translated to explain the “tears of wine” commonly observed in red wine. Hint: red wine is basically just water and ethanol.
I won’t explain this effect, but with some thought and experimentation, you should be able to describe the process using the principles taught above. You can check your conclusions with these great explanation videos by Applied Science and Dan Quinn
This project and blog post was inspired by the following paper:
L. Keiser, H. Bense, P. Colinet, J. Bico, and E. Reyssat. Marangoni Bursting: Evaporation-Induced Emulsification of Binary Mixtures on a Liquid Layer. PRL 118, 074504 (2017).